Retinopathy of prematurity (ROP) poses severe challenges for the survival and development of preterm infants. This condition, characterized by abnormal blood vessel growth in the retina, necessitates regular screening, which involves the use of mydriatics—eye drops designed to dilate the pupils. Traditionally, the administration of these drops in vulnerable infants has raised concerns regarding potential adverse effects on their delicate physiology. Recently, a randomized trial conducted by researchers led by Asimina Mataftsi, MD, PhD, has introduced a promising alternative: mydriatic microdrops. This article delves into the findings of the study, highlighting its implications for clinical practice and the safety of preterm infants.
The study, titled MyMiROPS, included 83 preterm infants, all of whom were at heightened risk for ROP. The infants were sourced from a tertiary medical center in Northern Greece between September 2021 and January 2023. Participants were required to meet specific criteria such as a gestational age below 32 weeks or a birth weight under 1,501 grams. The mean gestational age among the cohort was 29.7 weeks, with an average birth weight of 1,277 grams, creating a homogenous sample representative of critically ill preterm infants.
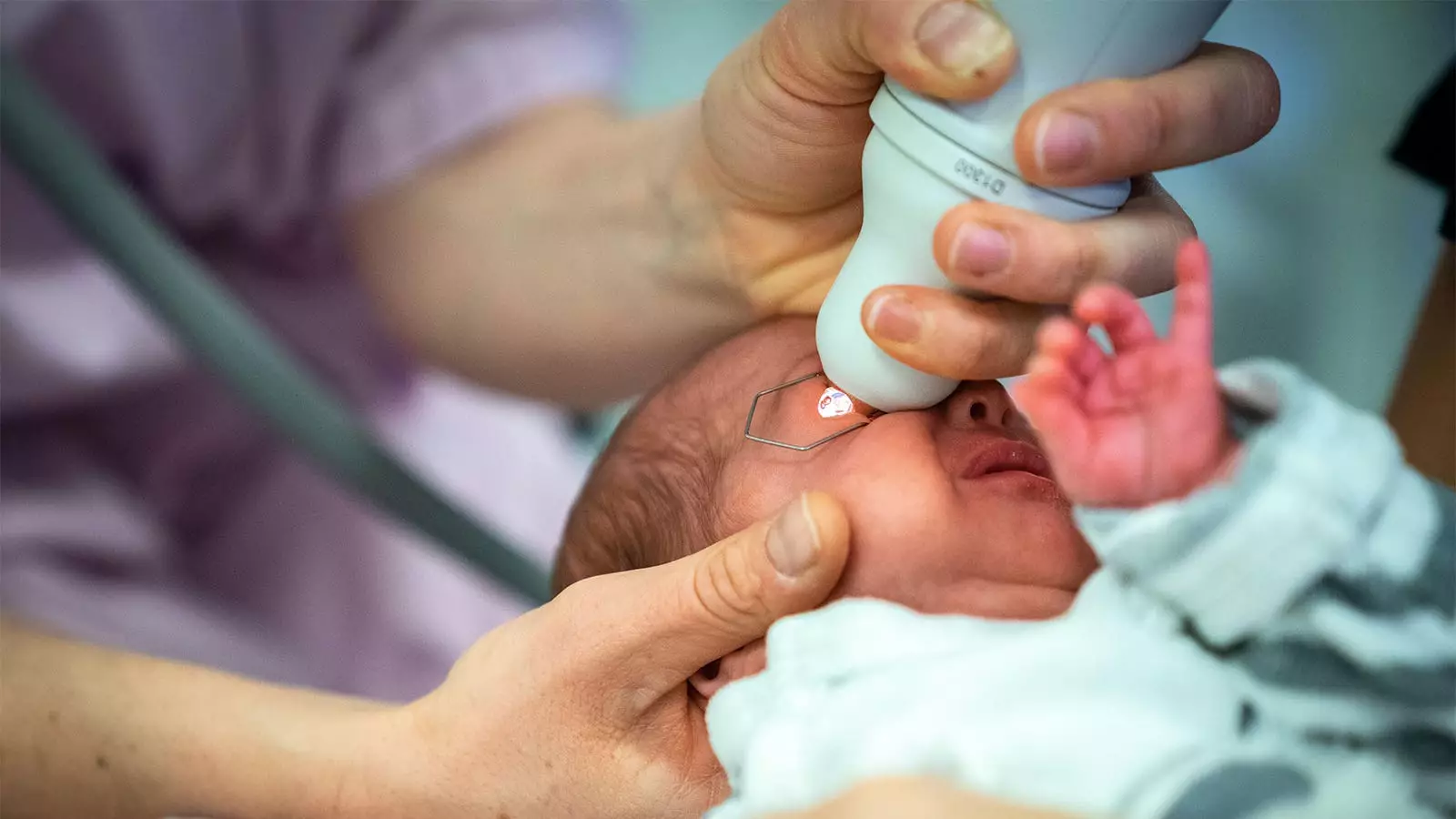
In this trial, infants were randomized to receive either microdrops or standard mydriatic drops. The dosages were significantly different—the microdrops constituted only 6.5 microliters, while the standard drops ranged between 28 to 34 microliters. A diluted mixture of phenylephrine and tropicamide was used in both groups to assess the mydriatic efficacy after set intervals of 45, 90, and 120 minutes.
The researchers found that mydriatic microdrops demonstrated superiority over standard drops for inducing significant pupil dilation at the 45-minute mark, with a mean difference of 0.12 mm. Furthermore, they maintained noninferiority at 90 and 120 minutes, suggesting that smaller doses can be equally effective in achieving the desired therapeutic effect without overexposing infants to potentially harmful side effects. The Bonferroni-corrected confidence intervals and statistical significance underscore the robustness of these findings.
Interestingly, the study revealed a concerning trend when standard drops were administered: infants exhibited lower oxygen saturation levels and an increase in hypertensive episodes compared to those receiving microdrops. These findings serve to highlight the systemic risks associated with traditional mydriatic interventions in this vulnerable population.
Historically, the use of standard mydriatics has been associated with significant systemic adverse events, particularly in preterm infants whose metabolic systems are still maturing. Previous literature indicated an increase in apnea events and other complications linked to routine eye examinations under standard protocols. The recent findings reiterate the necessity for critical evaluation of drug administration methods, particularly concerning our most fragile patients.
Mataftsi and her colleagues noted that their trial results support the use of microdrops, suggesting that these lower-dose alternatives may drastically reduce the risk of systemic repercussions. They acknowledged, however, that their study was not without limitations, specifically pointing out the absence of comprehensive safety outcome data for 30% of participants. This highlights the need for further research to entirely grasp the long-term implications of using microdrops in clinical practice.
This groundbreaking study signifies an essential shift in the approach to administering mydriatics for ROP screenings. The implications of these findings emphasize the potential for microdrops to mitigate systemic risks while preserving the efficacy of pupil dilation. Future studies will be vital in confirming these results across broader populations and different clinical scenarios to substantiate the protective benefits ascribed to microdrops.
The promise shown by mydriatic microdrops signals a potential paradigm shift in the management of ROP screening in preterm infants. By minimizing complications while ensuring efficacy, this innovative approach may not only improve clinical outcomes but also enhance the overall well-being of these vulnerable patients. Continued research and clinical application of this methodology could pave the way for safer practices in pediatric ophthalmology, ultimately serving to protect the most at-risk infants.
Leave a Reply